British physicist Ernest Rutherford once said: "The sciences are divided into two groups - physics and stamp collecting." He may have been somewhat categorical, but his the main idea is obviously true: physics is the most important of the sciences, helping to understand how the world works. Perhaps some interesting facts about physics encourage you to learn more about this science.
On the physical properties of atoms
- If you increase the diameter of an atom to the size of a football field, its nucleus will occupy the volume of a football.
- If you remove the free space of atoms, leaving only particles, a teaspoon of the resulting "substance" will weigh 5,000 billion kilograms. This is what neutron stars are made of.
About the physics of the sun
- In fact, the sun is white, because it radiates in all ranges. From the Earth, it appears yellow due to the fact that the Earth's atmosphere transmits well the rays from the yellow-red range, scattering the rays from the green-violet.
- In flight, many insects are guided by the sun. Since the sun is quite far from our planet (about 150,000,000 km), its rays can be considered parallel, and therefore, when an insect needs to move straight, it is enough for it to keep some identical angle to its rays. No one warned insects about the appearance of lamps - artificial light sources, therefore, in flight, they are guided by them, as by the sun. But the rays from the lamp diverge radially, and maintaining a specific angle to them brings insects: they spiral closer and closer to the light source until they crash into it (but even after that they do not understand their mistake). That's why mosquitoes flock to the lamps in the evening.
- If it were possible to accumulate the energy that the sun emits in a second, it would be enough to provide for all mankind for a million years.
About sound waves
- The first supersonic invention of mankind is the whip. Due to the fact that its tip moves faster than sound, a click is heard after the whip is swung.
- The denser the medium, the faster sound travels through it. For example, granite conducts it 10 times better than air. And if you clench your teeth wrist watch, their ticking will be much louder as the sound will propagate through the solid medium.
- Noise reaches 90-100 decibels. The same noise is produced by the factory floor.
On the physical properties of water
- Water conducts electricity only due to the ions of substances dissolved in it. Therefore, you can swim during a thunderstorm. True, only in distilled water.
- in different states of aggregation Water reflects light differently: snow reflects 75% of light, water 2%, and ocean ice 5%.
- At 4°C, water has its maximum density. It is thanks to this property that the layers in fresh water bodies are mixed: the water on the surface heats up, acquires maximum density, and sinks, while the water at the bottom cools, becomes less dense and rises to the surface.
About the atmosphere and its phenomena
- Almost all oxygen in the atmosphere is of biogenic origin. Before the advent of photosynthetic bacteria, the Earth's atmosphere was anoxic.
- Lightning has a temperature of 30,000 K, five times the surface temperature of the sun.
- Although the spectrum of the rainbow is continuous, it is believed that it consists of seven colors. Isaac Newton was the first to introduce this number, and at first he singled out only five colors (without orange and blue). However, later he added two more colors to make them seven, as the main tones in the musical scale.
On the history of physics
- The steam engine was first invented by the Greek scientist Heron of Alexandria in the 1st century AD. e. The machine, called aeolopile, was a sealed cauldron with two L-shaped tubes on the lid, on which a sphere rotated, which also had, in turn, two L-shaped tubes. Water was poured into the boiler, and the hole was plugged. When the boiler was heated, the water turned into steam and escaped through tubes on the sphere, rotating it. This mechanism did not find a practical application and was forgotten.
- In 1683, Christopher Wren bet 40 shillings that no one could explain the elliptical orbits of the planets for several months. Isaac Newton, hearing about this, accepted the challenge. The result was the book "Mathematical Principles of Natural Philosophy", in which he formulated his famous laws. True, he did not receive money for this, since it took several years to write the book.
- In 1897, Nikola Tesla invented a radio-controlled ship. Only after 100 years such toys began to be sold in stores.
- In Nazi Germany it was forbidden to receive Nobel Prize. Physicists James Frank and Max von Laue donated their medals to the Dane Niels Bohr. During the German occupation of Copenhagen, the chemist de Hevesy dissolved them in a mixture of hydrochloric and nitric acids, and at the end of the war he isolated what was hidden in it and gave it to the Swedish Academy of Sciences, where they re-made medals from it and handed it to Frank and von Laue.
Soviet physicist Lev Artsimovich said that science - The best way satisfaction of personal curiosity (while adding "at the expense of the state", but this is not the main thing). If all these interesting facts about physics warmed up your curiosity, do not stop at what you read: read more, find out how the world is tripled and, of course, look around!
1. How did life begin?
The appearance of the first living creature from inorganic material about 4 billion years ago is still shrouded in a veil of mystery. How did the relatively simple molecules contained in the primitive ocean form more and more complex substances? Why did some of them acquire the ability to absorb and transform energy, as well as to reproduce themselves (the last two properties are the hallmarks of living things)? At the molecular level, all these events are undoubtedly chemical reactions, and therefore the question of the origin of life should be considered within the framework of chemistry.
Chemists do not have the task of sorting through the myriad of scenarios of how things could have played out billions of years ago. Participated or not in the creation of self-replicating polymers (what DNA or protein molecules) inorganic catalysts, such as lumps of clay; or whether there was an “RNA world” in the distant past, in which the “cousin” DNA (RNA molecule) catalyzed the reactions of protein formation and appeared before other biopolymers.
It is necessary to test the validity of these hypotheses by conducting chemical reactions in a test tube. It has already been shown that some relatively simple chemicals can interact with each other to form the "building blocks" of such biopolymers as proteins and nucleic acids, i.e. amino acids and nucleotides, respectively. In 2009, a team of molecular biologists led by John Sutherland at the Laboratory of Molecular Biology in Cambridge demonstrated the possibility of obtaining nucleotides from molecules thought to be in the primordial ocean. Another group of researchers was interested in the ability of some RNAs to act as a catalyst, indicating the possible existence of an RNA world. Thus, step by step, it is possible to build a bridge from inanimate matter to self-reproducing living systems.
Now that we have learned a lot about our neighbors in solar system- about the presence of water on Mars, about hydrocarbon lakes on Titan, a moon of Saturn, about cold salty oceans, apparently hidden under an ice crust on Europa and Ganymede, satellites of Jupiter, and about many other things, - the question of the origin of terrestrial life forms has become part of global problem: what conditions are necessary for the origin of life and to what extent can its chemical bases vary? The range of questions has expanded even more over the past 15 years, during which more than 500 planets orbiting other stars have been discovered outside the solar system. These worlds, characterized by extraordinary diversity, have yet to be explored.
Such discoveries forced chemists to change their ideas about the chemical basis of life. So, for a long time it was believed that an absolutely necessary prerequisite for its origin is the presence of water. Today, scientists are not sure about this. Maybe instead of water, liquid ammonia, formamide, liquid methane or hydrogen will do in conditions of ultrahigh pressure in the upper layers of Jupiter? And why should the appearance of DNA, RNA and proteins be a necessary prerequisite for the formation of living systems? Artificial chemical structures have been created that are capable of self-reproduction without any nucleic acids. Perhaps just some molecular system that can serve as a matrix for copying itself is enough?
"Analysis modern forms life that exists on Earth, says Steven Benner of the Foundation for Applied Molecular Evolution in Gainesville, Fla., does not answer the question of whether the similarity of their fundamental features (the use of DNA and proteins) is due to the presence of a common ancestor or indicates universality of life. However, if we insist on the fact that we must remain within the already known facts then we're not going anywhere.
2 How are molecules formed?
The structure of molecules is the main subject studied by students of chemical specialties, while the graphical representation of molecules in the form of a set of circles and lines between them, corresponding to atoms and chemical bonds, is a pure convention, which is resorted to for convenience. There is still no agreement among scientists about which image of molecules is closest to reality.
In the 1920s German theoretical physicists Walter Heitler and Fritz London showed that a chemical bond can be represented using the equations of the newly emerging quantum physics, and the great American chemist Linus Pauling hypothesized that bonds are formed when overlapping in space of electron clouds of different atoms. An alternative theory by Robert Milliken and Friedrich Hund suggested that chemical bonds (with the exception of ionic bonds) are the result of overlapping atomic orbitals of the outer electrons of interacting atoms and the appearance of a molecular orbital enclosing these atoms. Here we fall into the sphere of competence of theoretical chemistry, which in fact is one of the areas of physics.
Education concept chemical bonds by overlapping atomic orbitals has become widespread, but not everyone believes that it is universal. The point is that the model structures of molecules built on its basis proceed from a number of simplifying assumptions and, thus, represent only an approximation. In fact, any molecule is a certain group of atomic nuclei immersed in an electron cloud, and the nuclei, figuratively speaking, compete with each other in “pulling it on themselves”, so that the whole structure “breathes” and changes. In the existing models, however, molecules are static formations, constructed taking into account only a part of the important properties.
Within the framework of quantum theory, one cannot give general definition chemical bond, which would correspond to the ideas of chemists about it, whose work ultimately comes down to the destruction of some chemical bonds and the formation of others. Currently, there are many ways to represent molecules as atoms bonded to each other. According to quantum chemist Dominick Marx of the University of Bochum in Germany, almost all of them are “good in some cases and completely unusable in others.”
Using computer simulations, today it is possible to predict the structure and properties of molecules with high accuracy, based on the principles of quantum mechanics - but only as long as the number of electrons participating in the formation of chemical bonds is relatively small. “Computational chemistry allows you to get the most realistic picture of what is happening,” says Marks. Computer simulation can be viewed as a virtual experiment that reproduces the course of a chemical reaction. But as soon as the number of electrons approaches several tens, numerical methods become powerless even with the most powerful computers. In this regard, the question arises: how, for example, to model complex biochemical processes occurring in a cell, or the behavior of multicomponent systems?
3. How do they affect external factors to our genes?
For a long time, the biological community was dominated by the idea that the individuality of each of us is determined by what genes we possess. However, it is equally important which ones we use. As elsewhere in biology, the latter is inextricably linked with the same chemistry.
The cells of the embryo in the earliest stages give rise to tissues of all possible types. As it develops, the so-called pluripotent stem cells differentiate and become specialized (blood cells, muscle, nerve cells, etc.). The latter retain their individual properties throughout the life of the organism. The formation of the human body is, in essence, the chemical transformations of chromosomes of stem cells, as a result of which the set of functioning and silent genes changes.
One of the revolutionary discoveries in the field of cloning and stem cell research is that these transformations are reversible. In the process of differentiation, cells do not inactivate some of the genes, maintaining only those that are needed now. They turn them off and keep them on alert. These genes can be activated, for example, under the action of certain environmental chemicals.
Particularly interesting and mysterious from the point of view of chemistry is the fact that the regulation of gene activity is carried out at the supraatomic and supramolecular levels, with the participation of entire groups of molecules interacting with each other. Chromatin - a complex between DNA and proteins that forms chromosomes - has a hierarchical structure. First, a double-stranded DNA molecule wraps around cylindrical particles consisting of special proteins - histones. Then the resulting “string of beads” fits in space into structures of a higher order. The cell strictly controls the folding process - its activity depends on where in the chromatin the given gene is located.
Restructuring of the chromatin structure occurs with the participation of specific enzymes that play a key role in cell differentiation. In embryonic stem cells, chromatin has a loose, disordered structure that thickens as genes are switched off during differentiation.
Chromatin structuring is accompanied by chemical transformations of both DNA and histones. They are joined by small molecules - markers that tell the cell which genes to turn off and which, on the contrary, to turn on. Such marks are called epigenetic factors because they do not affect the information contained in genes.
To what extent can mature cells be returned to a state of pluripotency? Will they have the properties of stem cells necessary for use in the regeneration of various tissues? The answer depends on the extent to which epigenetic marking can be reversed.
It is clear that in addition to the genetic language, in which many key instructions are written, cells use a completely different language from a chemical point of view - epigenetic. "A person may have a genetic predisposition to a disease, such as cancer, but whether or not it occurs depends on environmental factors acting through an epigenetic channel," says Bryan Turner of the University of Birmingham in England.
4. How does the brain form memory?
The brain can be likened to a chemical computer. The connection between the neurons that make up its "electric circuits" is carried out with the help of special molecules - neurotransmitters. They are released by one neuron, cross the synaptic cleft, bind to the receptors of another neuron, activate it, which activates the third, and so on. As a result, the nerve impulse propagates along the chain of neurons. The chemical nature of mental activity is manifested during memorization, when some information - a phone number or some event - is “imprinted” with the help of chemical signals in the form of various states of the nervous network. How is memory formed on the basis of chemical processes - both persistent and dynamic? What does it mean to remember, rethink, forget?
We only have answers to some questions. We know, for example, that an unconditioned reflex occurs in response to a certain cascade of biochemical processes leading to a change in the amount of neurotransmitters in the synapse. But even such simple process There are short term and long term components. A more complex phenomenon - the so-called declarative memory (for faces, places, etc.) - has a different mechanism and a different localization in the brain. The main player here is the receptor for the neurotransmitter dopamine, which is present in some neurons. Blocking it interferes with the preservation of declarative memory.
The formation of everyday declarative memory is often mediated by the so-called long-term potentiation, which involves dopamine receptors and is accompanied by an expansion of the region of the neuron that forms the synapse. With the expansion of this area, the connection of the neuron with its partners is strengthened, which manifests itself through an increase in the potential difference in the synaptic cleft under the action of a nerve impulse. The biochemistry of the process has become more or less clear in the last few years. It was found that actin filaments are formed inside the neuron - a protein that forms the internal framework of the cell, which determines its size and shape. The process can be interrupted if the stabilization of newly appeared filaments is prevented.
Long-term memory, once formed, is preserved due to the inclusion of genes encoding specific proteins. There is reason to believe that prions are among them. The latter can be in one of two alternative conformations. In the first case, prions are easily soluble, in the second, they are insoluble and transfer all protein molecules of a given type with which they happen to contact into this state. As a result, large prion aggregates are formed, which are involved in the development of various neurodegenerative disorders. It is this negative property of prions that has become an incentive for their identification and study. It was found that the aggregates perform in the body and useful features- they are involved in the preservation of memory.
There are still many blank spots in the history of how memory works, which biochemists will have to fill. How to interpret, for example, the concept of "remember something" if this "something" is stored in our memory? "This problem, which we are just beginning to solve, is very difficult to understand," says Nobel Prize-winning neuroscientist Eric Kandel of Columbia University.
Speaking about the chemical nature of the phenomenon of memory, one cannot but touch upon such an issue as the effect of pharmaceuticals on it. Some memory-enhancing substances are already known. Among them are sex hormones and synthetic compounds that act on the receptors for nicotine, glutamate, serotine, and other neurotransmitters. As neuroscientist Gary Lynch of the University of California notes, the fact that a long chain of events leads to the formation of long-term memory indicates that there are many targets in the body that “memory drugs” could target.
5. Is there a limit to the replenishment of the periodic system of elements?
Periodic table chemical elements, which hangs in a conspicuous place in every chemistry classroom, is constantly replenished. With the help of accelerators, nuclear physicists are getting new, superheavy elements with a larger number of protons and neurons in the nucleus than those 92 that exist in nature. They are not very stable, some fall apart within a fraction of a second after birth. But while such elements exist, they do not differ in their status from the rest: they have an atomic number and a mass number, and they have certain chemical properties. In the course of ingenious experiments, some properties of the atoms of seaborgium and hassium were investigated.
One of the goals of such studies is to find out whether there is a limit to the expansion of the periodic system, in other words, whether superheavy elements exhibit that periodicity in their behavior, which determines their position in the table. Already now we can say that some meet the specified requirements, others do not. In particular, their massive nuclei attract electrons with such force that they begin to move at a speed approaching the speed of light. As a consequence, the mass of electrons increases dramatically, which can lead to disorganization of the energy levels on which the Chemical properties elements, which means their position in the periodic table.
It is hoped that nuclear physicists will be able to find an island of stability - some area slightly beyond the current possibilities of obtaining synthetic elements, in which superheavy elements will live longer. However, the fundamental question remains about limit sizes. As shown by fairly simple quantum mechanical calculations, electrons can be held by a nucleus, the number of protons in which does not exceed 137. More complex calculations reject this limitation. “The Periodic Table does not end with number 137; in fact, it is not limited by anything,” says nuclear physicist Walter Greiner from the Goethe University in Frankfurt am Main, Germany. Experimental verification of this statement is still very far away.
6. Is it possible to create a computer based on carbon atoms?
Graphene-based computer chips - grids of carbon atoms - are potentially faster and more powerful than silicon. Obtaining graphene brought its creators the Nobel Prize in Physics for 2010, but the practical application of such "carbon" nanotechnology ultimately depends on whether chemists will be able to create structures with atomic precision. In 1985, fullerenes were synthesized, hollow closed network structures consisting entirely of carbon atoms, and six years later, carbon nanotubes with network walls were synthesized. It was expected that extremely strong electrically conductive structures would find a wide range of applications - from the production of ultra-strong composite materials based on them to the manufacture of tiny conductors and electronic devices, miniature molecular capsules and membranes for water purification. However, the full potential has not yet been realized. So, it is not possible to embed nanotubes in complex electronic circuits. AT recent times Graphite has become the center of attention of nanotechnologists.
It was possible to divide it into ultra-thin layers (this is graphene), from which ultra-miniature, cheap and durable electronic circuits can be made. Computer developers, using narrow, thinnest strips graphene, will be able to produce more advanced chips than silicon. "Graphene can be made into structures that can be easily connected to each other and embedded in electronic circuits," says Walt de Heer of the Georgia Institute of Technology. However, the etching method used in microelectronics is not suitable for creating graphene electronic circuits - it is too crude, so today graphene technology is a matter of thought, not real deeds. Perhaps the key to solving the problem of designing at the atomic level will be the use of organic chemistry methods - the combination of polyaromatic molecules from several hexagonal carbon rings, analogues of small fragments of a graphene network, with each other.
7. Is it possible to catch more solar energy?
Each sunrise reminds us that a person uses only a small fraction of the energy that our luminary provides. The main obstacle to its widespread use is the high cost of silicon solar cells. But the very life on our planet, ultimately supported by photosynthesis, which is carried out by green plants when they absorb solar energy, indicates that solar cells do not have to be highly efficient, it is enough that there are many of them (like leaves on trees) and they would be cheap.
"One of the most promising directions in developing ways to use solar energy – getting fuel,” says Devens Gust of Arizona State University. The easiest way to do this is by splitting with sunlight water molecules to form hydrogen and oxygen gas. Nathan S. Lewis and his collaborators at the California Institute of Technology are working on an artificial sheet of silicon nanowires that can do this splitting.
Recently, Daniel Nocera of the Massachusetts Institute of Technology announced the creation of a silicon membrane in which, with the participation of a cobalt-based photocatalyst, water molecules are actually split. Nosera estimates that one gallon (~3.8 l) of water can produce enough fuel to power the small house during the day.
The development of such technology is hampered by the lack of suitable catalysts. “A cobalt catalyst like Nosera used and new catalysts based on other metals are basically what we need, but they are too expensive,” Gast says. "Unfortunately, we don't know how the natural manganese-based photosynthetic catalyst works."
Gast and his colleagues intend to create molecular assemblies for artificial photosynthesis that mimic natural ones. They have already managed to synthesize a number of substances that will be included in one of these ensembles. But there are serious obstacles along the way. Organic molecules, similar to those used by nature, are unstable. Plants immediately replace them with new ones, and artificial leaves are not yet capable of this: they, unlike living systems, do not have biosynthetic mechanisms.
8. What is the best way to get biofuels?
Instead of developing technology to produce fuel using the energy of the Sun, is it better to use the ability of green plants to store energy and turn biomass into fuel? Biofuels such as ethanol are derived from corn and biodiesel from seeds, and these products already have a place in the market. But there is a danger that grain, which forms the basis of the human diet, will be used. This is especially undesirable for developing countries - the export of biofuels can be very profitable and leave the local population without food. In addition, to meet the current demand for fuel, vast areas now occupied by forests will have to be plowed up.
Thus, processing grain into fuel does not seem to be the best solution. One way out could be to use other, less valuable types of biomass. In the USA, there is enough waste from agriculture and the woodworking industry to satisfy one-third of the needs of transport in gasoline and diesel fuel.
The processing of such low grade biomass requires the breakdown of tough molecules such as lignin and cellulose. Chemists already know how to do it, but existing methods too expensive, energy-intensive and unsuitable for obtaining large quantities fuel.
John Hartwig and Aleksey Sergeev of the University of Illinois have recently overcome one of the biggest challenges in breaking down lignin - breaking the bonds between the carbon and oxygen atoms that bind benzene rings together. They used a nickel-based catalyst.
Obtaining from biomass fuel in industrial scale involves the processing of solid biomaterial on site in order to transport the resulting liquid through pipes. Here comes one serious problem- raw materials are heavily contaminated with various foreign impurities, and classical catalytic chemistry deals only with pure substances. “How, in the end, it will be possible to get out of the situation is not yet clear,” says Hartwig. One thing is clear: the problem is largely related to the field of chemistry, and its solution comes down to finding a suitable catalyst. “Almost all industrial processes involve the use of appropriate catalysts,” emphasizes Hartwig once again.
9. Is it possible to develop new ways to obtain drugs?
Chemistry is fundamentally a creative and at the same time practical science. It is engaged in obtaining molecules from which then you can create a variety of products - from materials with new properties to antibiotics that can destroy pathogens that are resistant to other drugs.
In the 1990s at the peak of popularity was combinatorial chemistry, when thousands of new molecules were obtained by random combination of "building blocks" and selected products with the desired properties. This direction, proclaimed at the beginning as the future of medicinal chemistry, soon lost its relevance, since the result turned out to be close to zero.
But, perhaps, combinatorial chemistry is waiting for a second birth. It will take place provided that a sufficiently wide range of molecules is obtained. certain type and a method was found for isolating microscopic amounts of the necessary substances from this mixture. Biotechnology is ready to help. For example, each molecule can be provided with a DNA-based barcode to facilitate its identification and isolation. An alternative approach would be to successively reject unsuitable candidates - a kind of Darwinian selection in vitro. To do this, it is possible to represent the amino acid sequence of a protein - a candidate for the role of a drug - in the form of a nucleotide sequence of a DNA segment and then, using the replication mechanism with its propensity for errors, to obtain more and more new variants that approach the ideal with each round of replication and selection. .
Other new methods rely on the inherent ability of some molecular fragments to connect to each other in a given sequence. Thus, the amino acid sequence of proteins is determined by the corresponding genes. Using this principle, chemists could in the future program molecules with the inherent ability to self-assemble. This approach has the advantage of minimizing the amount of by-products, which in turn reduces the energy intensity of the processes and the consumption of materials.
Currently, David Liu and his colleagues at Harvard University are trying to implement this idea. They attached to each building block of future molecules a short segment of DNA encoding a linker, and in addition they synthesized a molecule that moves along the DNA and sequentially adds monomeric units to the building block, guided by the instructions encoded in the DNA segment, a process similar to the synthesis of proteins in living cell. The Liu method can be useful for creating targeted drugs. "Many molecular biologists involved in pharmacology believe that macromolecules will play an increasing and then a major role in therapy," says Liu.
10. Is chemical monitoring of our body possible?
Recently, in chemistry, there has been an increasingly clear trend towards convergence with information technology, in particular, towards the use of chemical products for communication with living cells. The idea itself is not new: biosensors with chemical reactions were used to measure blood glucose as early as the 1960s, although they have only recently become widespread in diabetes monitoring with the advent of inexpensive portable devices. The scope of application of chemical sensors is wide: it is the detection of various harmful substances in food and water at very low concentrations, determination of the level of atmospheric pollution, and much more.
But there is another area - biomedicine - where the potential of chemical sensors can be fully revealed and bring invaluable benefits. For example, some gene products associated with a particular cancer begin to circulate in the bloodstream long before the onset of visible symptoms of the pathology, when conventional testing methods do not detect anything. Early identification of such chemical precursors of cancer will make it possible to make a more accurate diagnosis, and most importantly, to do it in a timely manner. Rapid genomic profiling will provide an opportunity to select an individual treatment regimen and reduce the likelihood of side effects.
Some chemists foresee the advent of an era of continuous, easy for the patient monitoring of a variety of biochemical markers of the state of the body. Such information can be useful to the surgeon right during the operation, it can be transferred to an automated drug administration system, etc. The implementation of these ideas depends on whether chemical methods selective identification of markers, even when they are present in the body in trace amounts.
What science is rich in interesting facts? Physics! Grade 7 is the time when schoolchildren begin to study it. So that a serious subject does not seem so boring, we suggest starting your studies with entertaining facts.
Why are there seven colors in the rainbow?
Interesting facts about physics can even touch the rainbow! The number of colors in it was determined by Isaac Newton. Even Aristotle was interested in such a phenomenon as a rainbow, and its essence was discovered by Persian scientists in the 13-14th century. However, we are guided by the description of the rainbow that Newton made in his Optics in 1704. He singled out the colors with a glass prism.
If you look closely at the rainbow, you can see how the colors smoothly flow from one to another, forming a huge number of shades. And Newton initially singled out only five main ones: purple, blue, green, yellow, red. But the scientist had a passion for numerology, and therefore he wanted to bring the number of colors to the mystical number "seven". He added two more colors to the description of the rainbow - orange and blue. So it turned out a seven-color rainbow.
Liquid form
Physics is around us. Interesting facts may surprise us, even when it comes to such a familiar thing as ordinary water. We are all used to thinking that a liquid does not have its own shape, even a school textbook on physics says this! However, it is not. The natural shape of a liquid is a sphere.

Eiffel tower height
What is the exact height of the Eiffel Tower? And it depends on the weather! The fact is that the height of the tower fluctuates by as much as 12 centimeters. This is due to the fact that in hot sunny weather the building heats up, and the temperature of the beams can reach up to 40 degrees Celsius. And as you know, substances can expand under the influence of high temperature.
Selfless Scientists
Interesting facts about physicists can be not only funny, but also tell about their dedication and dedication to their favorite work. While studying the electric arc, physicist Vasily Petrov removed upper layer skin on the fingertips to feel the weak currents.
And Isaac Newton introduced a probe into his own eye to understand the nature of vision. The scientist believed that we see because light presses on the retina.

quicksand
Interesting facts about physics can help to understand the properties of such an entertaining thing as quicksand. They represent a Human or animal cannot completely sink into quicksand due to its high viscosity, but it is also very difficult to get out of it. To get your foot out of the quicksand, you need to make an effort comparable to lifting a car.
You can’t drown in it, but life is dangerous from dehydration, the sun, and hot flashes. If you get into quicksand, you need to lie on your back and wait for help.

supersonic speed
You know what was the first device that overcame the Common Shepherd's Whip. The click that frightens the cows is nothing more than a pop when overcoming strong blow the tip of the whip moves so fast that it creates a shock wave in the air. The same thing happens with an aircraft flying at supersonic speeds.
Photonic spheres
Interesting facts about the physics and nature of black holes are such that sometimes it is simply impossible to even imagine the implementation of theoretical calculations. As you know, light is made up of photons. Falling under the influence of the gravity of a black hole, photons form arcs, areas where they begin to orbit. Scientists believe that if you put a person in such a photon sphere, he will be able to see his own back.

Scotch
It is unlikely that you unwound tape in a vacuum, but scientists in their laboratories did it. And they found that when unwinding, a visible glow and X-rays appear. The power of X-ray radiation is such that it even allows you to take pictures of parts of the body! Why this happens is a mystery. A similar effect can be observed upon the destruction of asymmetric bonds in a crystal. But here's the problem - there is no crystalline structure in scotch tape. So scientists will have to come up with another explanation. Do not be afraid to unwind the tape at home - no radiation occurs in the air.
Experiments on humans
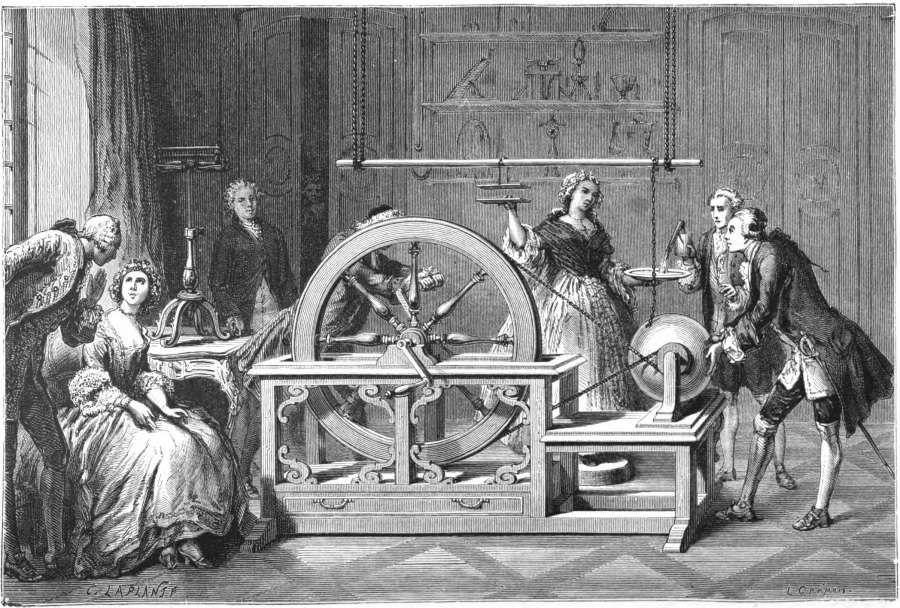
In 1746, the French physicist and part-time priest Jean-Antoine Nollet explored nature electric current. The scientist decided to find out what is the speed of the electric current. Here's just how to do it in a monastery...
The physicist invited 200 monks to the experiment, connected them with iron wires and discharged a battery from the recently invented Leyden jars into the poor fellows (they are the first capacitors). All the monks reacted to the blow at the same time, and this made it clear that the speed of the current was extremely high.

Genius Loser
Interesting facts from the life of physicists can give false hopes to underachieving students. There is a legend among negligent students that the famous Einstein was a real loser, did not know mathematics well and generally flunked his final exams. And nothing, became world We hasten to disappoint: Albert Einstein began to show remarkable mathematical abilities as a child and had knowledge that far exceeded the school curriculum.
Perhaps the rumors about the poor performance of the scientist arose because he did not immediately enter the Zurich Polytechnic School. Albert brilliantly passed the exams in physics and mathematics, but in other disciplines he did not score the required number of points. Having improved his knowledge in the necessary subjects, the future scientist successfully passed the exams the following year. He was 17 years old.
Birds on a wire
Have you noticed that birds love to sit on wires? But why don't they die from electric shock? The thing is that the body is not a very good conductor. The bird's paws create a parallel connection through which a small current flows. Electricity prefers wire, which is the best conductor. But as soon as the bird touches another element, for example, a grounded support, electricity rushes through its body, leading to death.

Hatches against fireballs
Interesting facts about physics can be remembered even while watching Formula 1 city races. Sports cars move at such high speeds that a low pressure is created between the bottom of the car and the road surface, which is enough to lift the hatch cover into the air. This is exactly what happened at one of the city races. The manhole cover collided with the next car, a fire broke out and the race was stopped. Since then, manhole covers have been welded to the rim to avoid accidents.
natural nuclear reactor
One of the most serious branches of science is nuclear physics. There are interesting facts here as well. Did you know that 2 billion years ago, a real natural nuclear reactor operated in the Oklo region? The reaction proceeded for 100,000 years until the uranium vein was depleted.
An interesting fact is that the reactor was self-regulating - water entered the vein, which played the role of a neuron moderator. With the active course of the chain reaction, the water boiled away, and the reaction weakened.
Page 1
The interesting F3NO molecule also has a tetrahedral structure.
These interesting molecules can either donate a carboxyl proton or add another proton to the amino group.
Xenon forms a number of interesting molecules and ions with fluorine and oxygen. Indicate which atoms in these Lewis structures have nonzero formal charges.
When trying to use NOE measurements for more interesting molecules than those given in the previous section, we will run into some difficulties. Probably, a large number of interacting protons will make it impossible to calculate internuclear distances. The assumption of equal correlation times for all internuclear vectors, on which such calculations are based, most likely does not hold for large molecules at all, and we should not forget this. In order to succeed in determining the structures of complex molecules, we must partially forget about the two main principles from Sec. We will assume that the observed NOE value reflects the relative proximity of the nuclei, and we must bear in mind that in some cases our conclusions may be incorrect.
Progress made over last years in the field of modern structural chemistry, is reduced mainly to the determination of the structures of a number of particularly interesting molecules and crystals.
At the same time, the gas chromatography method for studying adsorption is distinguished by its high sensitivity, which makes it possible to study the region of small fillings, and the ability to work on commercial equipment in wide area temperatures and, therefore, to study adsorption interactions a large number of interesting molecules of various structures. However, the approximation of the theory of non-linear equilibrium chromatography is used in this case. Comparison with static studies shows that usually the criterion for sufficient proximity to equilibrium conditions in the column during developing chromatography is, firstly, the coincidence of the blurred peak boundary for different samples (from zero to the isotherm inflection point) and, secondly, the verticality of the opposite peak boundary .
In the [CIO2] anion, the OC1O angle is 110 5, the chlorine-oxygen bond length is 156 pm. An interesting molecule with a similar angular structure is C1C2, in which the OC1Q angle is 117 4, and the C1 - O distance is 147 pm. This molecule is unusual, because although it is paramagnetic, but in contrast to NO2, dimers (see p. Since the C1 - O bonds in it are noticeably shorter than the bonds in the chloride ion, the order of the bonds must be greater. The simplest way to describe the formation of bonds is to start from structures of sulfur dioxide and consider that the additional electron is in the antibonding orbital.
We now want to talk about one of the most interesting molecules, the benzene molecule, the diagram of which is shown in Fig. It includes six very symmetrically arranged carbon and hydrogen atoms. Each dash in the diagram represents a pair of electrons with opposite spins dancing a covalent bond dance. Each hydrogen atom brings one electron into play, and each carbon atom four, for a total of 30 electrons in play.
Thus, isooctane gives only two primary products: greg-butylcation and isobutylene. Here, several important points should be noted that make isooctane the most interesting molecule from the point of view of studying the carbonium-ion mechanism of the transformation of alkanes.
The spectra of hardly volatile halides were considered above. various elements, however, oxides are even more difficult to volatilize. One of the first objects of research was boron oxide, but so far the problems of the structure and spectra of this very interesting molecule have not been resolved, so let us dwell in more detail on the history and research technique.
Now, using the simplest molecule, the molecular hydrogen ion H, as an example, we will first identify the most essential features of the theory of molecular structure, and then we will discuss more complex and chemically more interesting molecules.
Comparing the proton chemical shifts 1 6 8 13 - b c-methano annulene (31) and the data for 1 6-methano annulene, we can conclude that there is no ring current in 31, the existence of which can be assumed based on the number of jt - electrons. As the study of molecular models shows, between the centers 6, 7, 8 and 13, 14, 1 there is a strong twisting of carbon-carbon bonds, which makes it so difficult to effectively overlap the 2r orbitals of carbon that for the first time a compound has a number of n-electrons that exactly corresponds to Hückel's aromaticity rule, exhibits olefinic properties. We will return to this interesting molecule later.
However, any proposed structure must be verified by comparing the spectrum predicted on its basis with the experimental one. In this case, two circumstances should be pointed out. For such a complex molecule as [Fe3(CO)12] to have a relatively simple spectrum, its symmetry must be fairly high. The weakness of the bands thus seems to be an argument against the presence of ketone bridges in the molecule. However, then the question of what the weak bands can be attributed to becomes unclear. Obviously, further studies of this interesting molecule are needed.
Pages: 1
If you think physics is a boring and unnecessary subject, then you are deeply mistaken. Our entertaining physics He will tell you why a bird sitting on a power line wire does not die from electric shock, and a person who has fallen into quicksand cannot drown in them. You will find out whether there really are no two identical snowflakes in nature and whether Einstein was a loser at school.
10 fun facts from the world of physics
Now we will answer the questions that concern many people.
Why does a train driver back up before moving off?
The reason for this is the static friction force, under the influence of which the train cars are standing still. If the locomotive simply moves forward, it may not move the train. Therefore, he slightly pushes them back, reducing the static friction force to zero, and then gives them acceleration, but in the other direction.
Are there identical snowflakes?
Most sources claim that in nature there are no identical snowflakes, since several factors influence their formation at once: humidity and air temperature, as well as the snow flight path. However, entertaining physics says: you can create two snowflakes of the same configuration.

This was experimentally confirmed by the researcher Karl Liebbrecht. Having created absolutely identical conditions in the laboratory, he obtained two superficially identical snow crystals. True, it should be noted that their crystal lattice was still different.
Where is the largest reservoir of water in the solar system?
Never guess! The most voluminous storage of water resources in our system is the Sun. The water is in the form of steam. Its highest concentration is noted in places that we call "spots on the Sun." Scientists even calculated that in these regions the temperature is one and a half thousand degrees lower than in the rest of our hot star.
What invention of Pythagoras was created to combat alcoholism?
According to legend, Pythagoras, in order to limit the use of wine, made a mug that could be filled with an intoxicating drink only up to a certain mark. It was worth exceeding the norm even by a drop, and the entire contents of the mug flowed out. This invention is based on the law of communicating vessels. The curved channel in the center of the mug does not allow it to be filled to the brim, "relieving" the container of all the contents in the case when the liquid level is above the channel bend.

Is it possible to turn water from a conductor into an insulator?
Entertaining physics says: you can. Current conductors are not the water molecules themselves, but the salts contained in it, or rather their ions. If they are removed, the liquid will lose its ability to conduct electricity and become an insulator. In other words, distilled water is a dielectric.

How to survive in a falling elevator?
Many people think: you need to jump at the moment the cabin hits the ground. However, this opinion is incorrect, since it is impossible to predict when a landing will occur. Therefore, entertaining physics gives another advice: lie on your back on the floor of the elevator, trying to maximize the area of \u200b\u200bcontact with it. In this case, the impact force will not be directed to one part of the body, but will be evenly distributed over the entire surface - this will significantly increase your chances of survival.

Why does a bird sitting on a high voltage wire not die from electric shock?
The bodies of birds do not conduct electricity well. By touching the wire with its paws, the bird creates a parallel connection, but since it is not the best conductor, the charged particles do not move through it, but along the cable cores. But as soon as the bird comes into contact with a grounded object, it will die.

The mountains are closer to the source of heat than the plains, but on their peaks it is much colder. Why?
This phenomenon has a very simple explanation. The transparent atmosphere freely passes the sun's rays without absorbing their energy. But the soil perfectly absorbs heat. It is from it that the air then warms up. Moreover, the higher its density, the better it retains the thermal energy received from the earth. But high in the mountains, the atmosphere becomes rarefied, and therefore less heat “lingers” in it.

Can quicksand suck?
In films, there are often scenes where people "drown" in quicksand. AT real life- says entertaining physics - this is impossible. You won’t be able to get out of the sandy swamp on your own, because in order to pull out only one leg, you will have to make as much effort as it takes to lift a medium-weight car. But you also cannot drown, because you are dealing with a non-Newtonian fluid.

Rescuers advise in such cases not to make sudden movements, lie with your back down, spread your arms to the sides and wait for help.
Does nothing exist in nature, see the video:
Amazing cases from the life of famous physicists
Outstanding scientists, for the most part, are fanatics of their field, capable of anything for the sake of science. So, for example, Isaac Newton, trying to explain the mechanism of perception of light by the human eye, was not afraid to experiment on himself. He inserted a thin, carved ivory probe into the eye, simultaneously pressing on the back of the eyeball. As a result, the scientist saw rainbow circles in front of him and proved in this way: the world we see is nothing but the result of light pressure on the retina.

Russian physicist Vasily Petrov, who lived in early XIX century and engaged in the study of electricity, cut off the top layer of skin on his fingers to increase their sensitivity. At that time, there were no ammeters and voltmeters that could measure the strength and power of the current, and the scientist had to do it by touch.
The reporter asked A. Einstein if he writes down his great thoughts, and if he does, then where - in a notebook, notebook or special file. Einstein looked at the reporter's bulky notepad and said, "My dear! Real thoughts come so rarely to the head that it is not difficult to remember them.
But the Frenchman Jean-Antoine Nollet preferred to experiment on others. Conducting an experiment in the middle of the 18th century to calculate the speed of transmission of electric current, he connected 200 monks with metal wires and passed voltage through them. All participants in the experiment twitched almost simultaneously, and Nolle concluded: the current runs through the wires, well, oh, very quickly.
Almost every student knows the story that the great Einstein was a loser in his childhood. However, in fact, Albert studied very well, and his knowledge of mathematics was much deeper than the school curriculum required.

When the young talent tried to enter the Higher Polytechnic School, he scored the highest score in the core subjects - mathematics and physics, but in other disciplines he had a slight shortage. On this basis, he was denied admission. On the next year Albert showed excellent results in all subjects, and at the age of 17 he became a student.
Take it, tell your friends!
Read also on our website:
show more